Cell-free expressed OprF proteoliposomes as effective vaccine against Pseudomonas aeruginosa ?
Synthelis’ cell-free liposome-based expression system was applied in the development of an OprF vaccine against Pseudomonas aeruginosa. The complete OprF outer membrane protein of P. aeruginosa was used as the immunogen and was expressed in its native form in liposomes.
The resulting OprF proteoliposomes were fully characterized and their ability to induce an immunogenic response in mice was tested. The results showed the attractive features of the developed recombinant vaccine and its effectiveness in inducing a protective humoral response against P. aeruginosa, producing neutralizing antibodies that prevented or cured P. aeruginosa infections. The developed vaccine shows great promise as a candidate for human use.
Pseudomonas aeruginosa: major cause of nosocomial infections
Pseudomonas aeruginosa is a Gram-negative bacterium and a major responsible for nosocomial infections and pneumonia in hospital environment, frequently associated with the use of hospital devices such as catheters, central lines and ventilators [1]. P. aeruginosa also colonizes the respiratory tract of patients with obstructive lung diseases, resulting in chronic infections that can be lethal [2]. Furthermore, P. aeruginosa is known to present a high capacity to develop resistances to antibiotics [3]. Together, all these aspects lead to the urgent need for new antimicrobial strategies that allow to reduce the burden of P. aeruginosa infections and the associated morbidity and mortality. Considering the ability of this agent to develop or acquire new resistance mechanisms, the use of a vaccine to prevent infections constitutes a valuable and clever alternative approach to surpass the current problem of P. aeruginosa antibiotic resistance [4,5].
The outer membrane protein F (OprF): a good target for a vaccine development
The outer membrane proteins of Gram-negative bacteria are useful targets for the development of vaccines since most of them expose epitopes of the bacterial surface that can be recognized by the host immune system. In P. aeruginosa, the outer membrane protein F (OprF) seems particularly suitable for this purpose, as it is highly conserved and antigenically related among the serotypes of P. aeruginosa [6]. Moreover, it participates in diverse infection events such as biofilm formation, outer membrane vesicles biogenesis, binding and adhesion to host cells, involvement in quorum-sensing response and sensor of host immune activation through IFN-γ binding [7,8]. Structurally, OprF is a porin, presenting closed and open channel conformations. In the closed conformation, the N-terminal part spans eight times through the membrane, whereas the C-terminal part is located in the periplasm [9,10]. In its open conformation, the C-terminal part is located in the outer membrane, forming with the N-terminal part an integral membrane protein [10]. The closed channel conformation is the most represented (95%) and has a structural role in the bacterial cell wall.
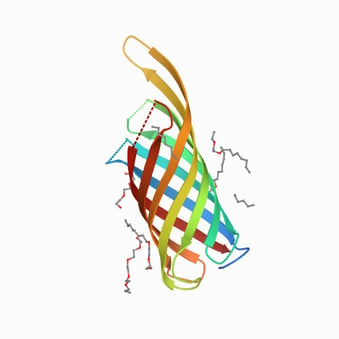
OprF protein structure
Cell-Free protein synthesis, a solution for OprF production
In spite of the favourable characteristics of OprF to be used as an immunogen in a vaccine, it also presents some problems, since it is difficult to develop a vaccine against a multispanning membrane protein, in its native conformation. Furthermore, it exhibits cellular toxicity on induction and gives low yields of correctly folded recombinant protein [11]. To surpass these limitations, a cell-free protein synthesis (CFPS) system can be used, in which the expression of membrane proteins is not impaired by their potential lethality [12] and can easily be adapted for large-scale protein production [13]. In the presence of liposomes or nanodiscs, cell-free systems allow the membrane protein to be translated and spontaneously inserted in the liposomal membrane in a one-step reaction [14]. In addition, CFPS systems enable the reconstitution of the complete membrane protein in its active form, presenting the native antigens [12,15].
The development and characterization of a OprF-based vaccine against P. aeruginosa was reported in 2021 [16] by using E. coli-based cell-free expression, in which the full-length OprF protein of P. aeruginosa was reconstituted in proteoliposomes, in the presence of synthetic liposomes. Fusion of the recombinant proteoliposomes into preformed tethered lipid bilayer membranes resulted in the incorporation of OprF in its native closed conformation and also, for a large proportion (36%), in its rare active open conformation, which resulted in the formation of mega-pores across the liposomal membrane.
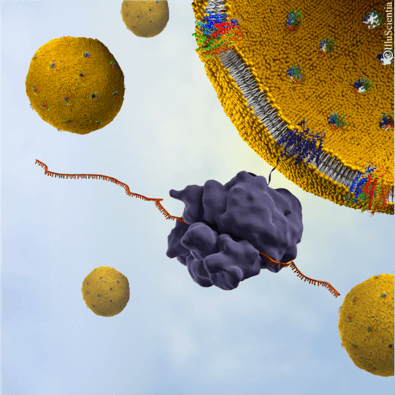
Illustration of liposome-based Synthelis expression system
Features of the OprF proteoliposomes
First, the authors optimized the lipid composition of the liposomes, which allowed to obtain protein yields from 0.5 to 1 mg/ml. The subsequent two-step purification process enabled to get highly pure (around 95% purity) OprF proteoliposomes. Most of the OprF proteins were shown to be embedded into the liposomal membrane in the correct orientation and in accordance with the two transmembrane topologies (closed and open channel conformers). Furthermore, the formation of large OprF pores in the liposomes was observed, with an average diameter of 9.5 ± 4 nm, which could be due to OprF oligomerization.
The OprF porin activity was assessed and it was shown that the porin was permeable to KCl. The influence of interferon-gamma (IFN-γ) was evaluated on the ion channel activity, since it is known that IFN-γ binds OprF and triggers cellular responses, increasing the virulence of P. aeruginosa [8]. A permanent inhibitory effect was observed, in response to increasing concentrations of IFN-γ. Furthermore, IFN-γ addition to the OprF proteoliposomes affected OprF secondary structure, producing disordered structures and impairing its activity. This interaction can be a mechanism that allows the bacteria to escape from the host immune system by blocking its OprF pore.
The storage stability of the OprF proteoliposomes was assessed, showing that they could be stored at 4 °C or lower temperatures, for at least two weeks, without protein degradation, therefore meeting the storage conditions necessary of vaccine preservation.
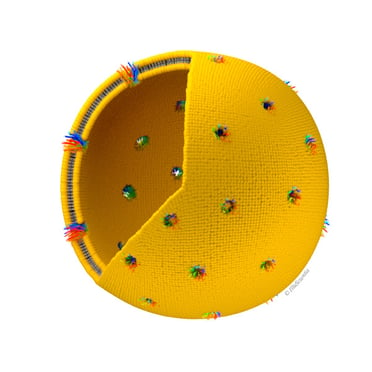
Image of proteoliposome
Efficient immunization with OprF proteoliposomes
Finally, the use of the developed OprF proteoliposome as a potential candidate for a vaccine against P. aeruginosa was evaluated, by immunizing mice with the proteoliposomes and then inoculating them with a lethal dose of the mucoid CF isolate CHA pathogenic strain of P. aeruginosa, with the results showing that the immunization conferred a 90% protection rate. In addition, the OprF proteoliposomes were able to induce the production of neutralizing antibodies, since sera from immunized mice contained polyclonal antibodies capable of recognizing both the recombinant OprF protein in the liposomes and the native protein from bacterial lysates.
The vaccine response of the OprF proteoliposomes against a mucoid strain of P. aeruginosa was higher than the ones reported for other vaccine strategies based on the use of partial OprF protein [17-19], demonstrating the advantages of using the OprF full-length protein, expressed in a cell-free system, without requiring the inclusion of other immunogens. This procedure allowed fostering the occurrence of the rare open-channel conformer, exposing new native epitopes which exist neither in the closed conformer nor in the partial OprF protein. Thus, the OprF proteoliposomes may be a better candidate for a vaccine against P. aeruginosa.




Leave a Reply
Want to join the discussion?Feel free to contribute!